Abstract
Clonal hematopoiesis (CH) is a condition wherein hematopoietic stem cells (HSCs) acquire mutations, often in presumed leukemia driver genes such as in DNMT3A, leading to clonal dominance and disproportionate contribution to peripheral blood without overt hematologic abnormalities (Jaiswal et al. 2014). In large cohorts of patients with solid tumors including colorectal cancer, presence of CH is associated with inferior overall survival due to progression of primary malignancy (Coombs et al. 2017). While potential involvement of CH in the pathogenesis of solid tumors driven by unrelated genetic alterations has far-reaching translational implications, the mechanistic understanding of this relationship is lacking.
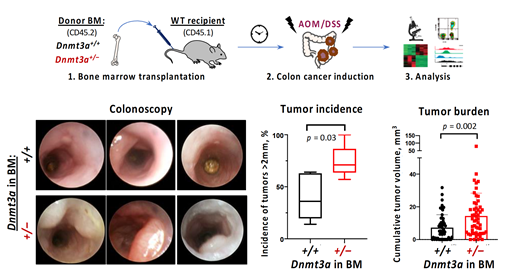
Colorectal cancer is a leading cause of cancer deaths worldwide and can arise from preceding inflammatory bowel disease. More than 20% of patients with colon cancer have detectable CH, which is notably more prevalent than in age-matched general population. We hypothesized that bone marrow-derived cells harboring alterations in DNMT3A, the most commonly disrupted gene in CH (up to 40% of cases), contribute to the pathogenesis of colorectal cancer. To address this, we combined a bone marrow transplantation-based model of CH driven by heterozygous loss of Dnmt3a with a well-established induction of colitis-associated colon cancer (CAC) by azoxymethane (AOM, a carcinogen) and dextran sodium sulfate (DSS, a colitogen). High resolution colonoscopy demonstrated increased colon wall opacity, visible bleeding, numerous fibrin patches and earlier onset of tumor formation with higher penetrance in animals with partial ablation of Dnmt3a in the hematopoietic compartment. Heightened colon pathology was reflected by modified murine endoscopic index of CAC severity (MEICS) (Fung and Putoczki 2018) (MEICS scores 7.5±2.0 in WT vs 10.0±3.2 in Dnmt3a +/-, p=0.05, n=14,13). We observed elevated incidence of tumors >2mm in diameter (40.2±22.2% in WT vs 74.2±15.7% in Dnmt3a +/-, p=0.03, in 5 independent trials) and a higher tumor burden in Dnmt3a +/- chimerae (7.4±7.8 mm 3 in WT vs 14.5±13.9 mm 3 in Dnmt3a +/-, p=0.0017, n=53,55) at end point. Histopathological analysis revealed marked immune infiltration, extensive ulceration and dysplasia of colonic epithelium, and more frequent adenocarcinoma formation with occasional submucosal invasion (histology score 20.2±4.4 in WT vs 24.6±6.9 in Dnmt3a +/-, p=0.03, n=10,9). To uncover signaling pathways deregulated by experimental Dnmt3a-driven CH, we surveyed the auto- and paracrine stimulatory loops by multiplex cytokine/chemokine assays and profiled transcriptomes of colon tumors by bulk RNA-seq. These studies identified gene signatures commonly associated with colon carcinogenesis including accentuated epithelial-to-mesenchymal transition (gene set enrichment analysis (GSEA) NES=2.42, padj=0.0096), Wnt/b-catenin (NES=2.07, padj=0.0096), and Vegf/angiogenesis (NES=1.83, padj=0.0096). Elevated proliferation (Ki67) and increased vascularization (CD31) were validated by immunohistochemistry. These aspects of tumor pathophysiology along with the composition and functional characteristics of the tumor-infiltrating leukocytes derived from animals with and without hematopoietic-specific Dnmt3a partial loss are being further investigated. Preliminary data showed bone marrow-derived macrophages (BMDMs) from Dnmt3a +/- chimera exhibit impaired ability to phagocytose syngeneic MC38 colon tumor cells, compared to WT.
Our study provides new insights into the non-tumor-cell-autonomous mechanisms of carcinogenesis effected by alterations in the hematopoietic system and into the interplay between the immune system and the aggressive phenotype of colorectal cancer. Our findings may aid the development of novel therapeutic options to improve outcomes in solid tumor patients with CH.
Bowman: Mission Bio: Honoraria, Speakers Bureau. Levine: Ajax: Membership on an entity's Board of Directors or advisory committees; Isoplexis: Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria; Prelude: Membership on an entity's Board of Directors or advisory committees; Auron: Membership on an entity's Board of Directors or advisory committees; Mission Bio: Membership on an entity's Board of Directors or advisory committees; C4 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Zentalis: Membership on an entity's Board of Directors or advisory committees; Imago: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Lilly: Honoraria; QIAGEN: Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy; Astellas: Consultancy; Janssen: Consultancy; Incyte: Consultancy; Roche: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal